
Published in 2024, “The Role of Artificial Intelligence in the Development of Anticancer Therapeutics from Natural Polyphenols” presents groundbreaking insights into how AI is reshaping cancer treatment. Natural polyphenols, compounds found in everyday foods like fruits, tea, and coffee, hold incredible anticancer properties. However, despite their potential, traditional drug development is often slow and costly. Now, artificial intelligence is stepping in to revolutionize how we unlock the therapeutic power of polyphenols like resveratrol and curcumin.
By using AI in drug discovery, researchers are cutting development time, increasing efficiency, and uncovering new ways to treat cancer. This article dives deep into how AI is applied to these natural compounds, focusing on how it helps improve the bioavailability of polyphenols, predict their biological activity, and enhance their effectiveness against cancer.
The Role of Artificial Intelligence in the Development of Anticancer Therapeutics from Natural Polyphenols
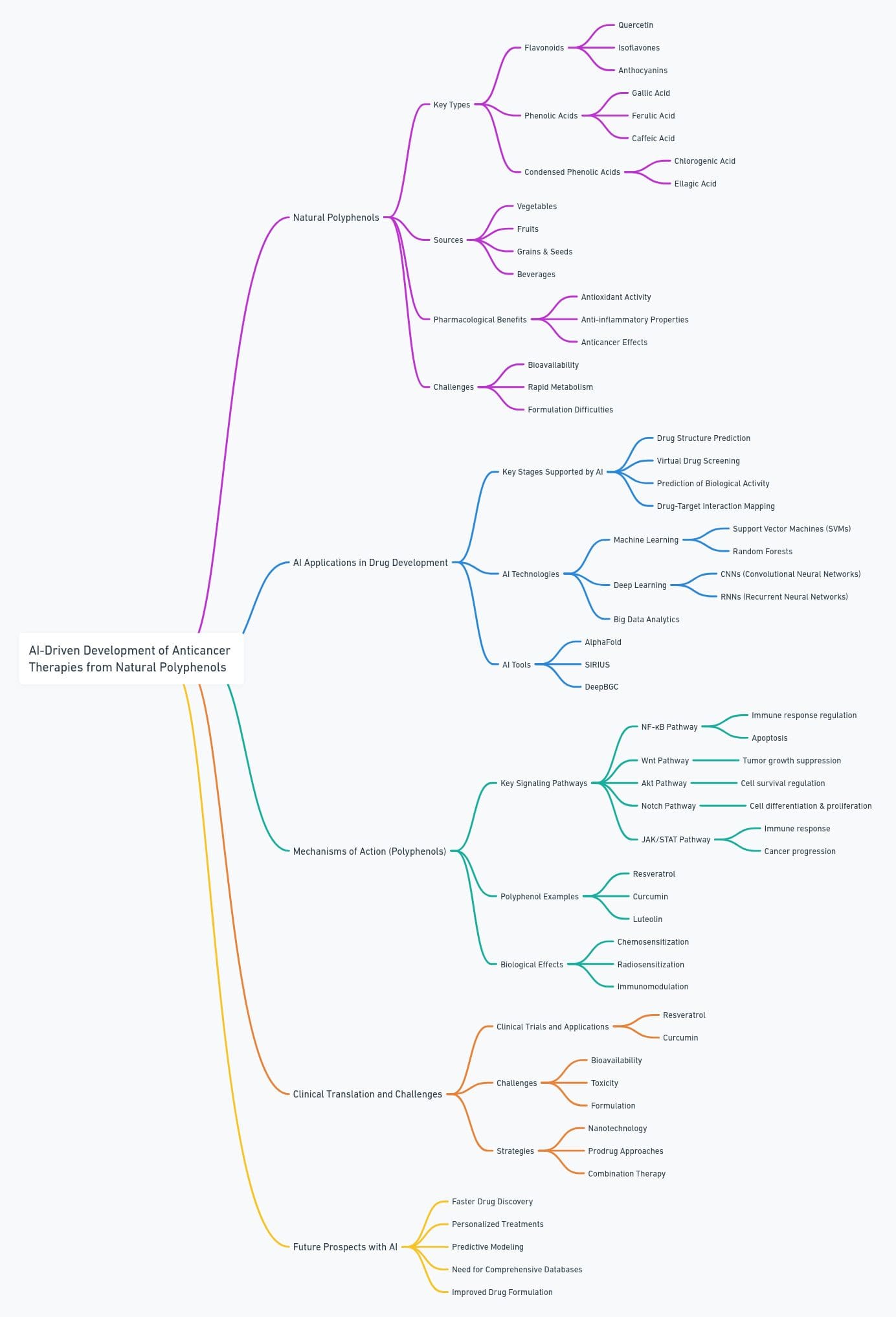
This summary covers the central themes of the article on AI’s application in anticancer drug discovery involving natural polyphenols.
Summary
- 🌿 Natural Polyphenols’ Potential: Natural polyphenols found in daily diets, such as resveratrol, curcumin, and EGCG, show promising anticancer properties, but traditional drug development methods are slow and expensive.
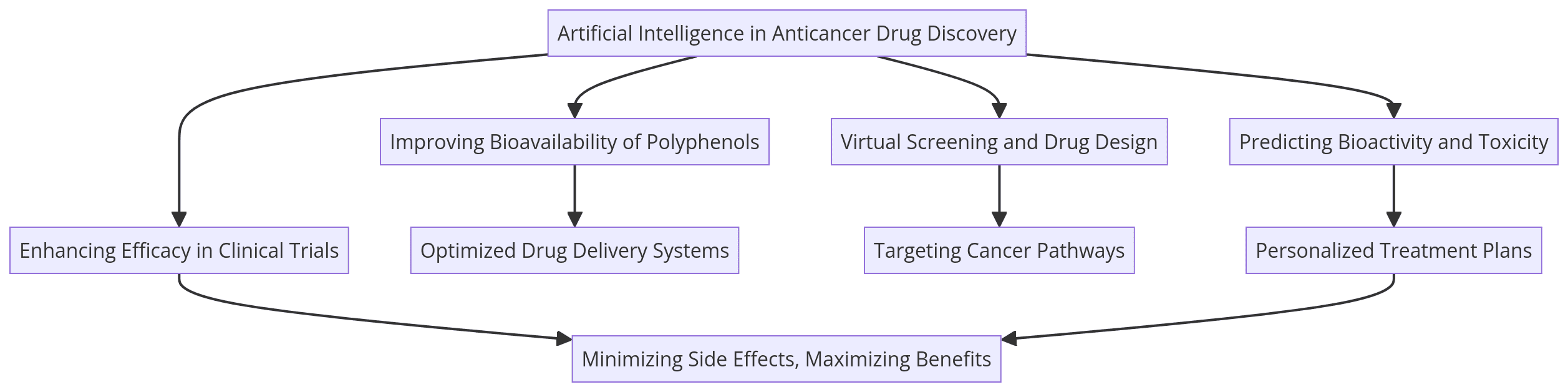
- 🤖 AI’s Role in Drug Discovery: Artificial intelligence (AI) accelerates the identification and development of natural polyphenol-based anticancer drugs by streamlining key processes like drug structure identification, virtual screening, and bioactivity prediction.
- ⚙️ Mechanisms of Action: Polyphenols interact with multiple cancer-related signaling pathways, including NF-κB, Wnt, Akt, Notch, and JAK-STAT, leading to tumor suppression through mechanisms like cell cycle arrest, apoptosis, and reduced proliferation.
- 💻 Virtual Screening & AI: AI-driven virtual screening tools allow researchers to evaluate vast numbers of compounds quickly, identifying those most likely to be effective in cancer treatment.
- 🧬 Omics and AI Integration: Combining AI with omics data (genomics, proteomics, and metabolomics) helps identify potential drug targets and pathways affected by polyphenols, accelerating the drug discovery process.
Insights Based on Numbers
- 🧪 Clinical Trials: Multiple polyphenols have progressed to phase II and III clinical trials, demonstrating improved cancer therapy outcomes when combined with conventional treatments.
••📊AI Efficiency: AI reduces the time and cost of drug development significantly, with structure-based and ligand-based virtual screenings enhancing accuracy and speed
Explanatory Q&A
1. How can artificial intelligence further improve the bioavailability of natural polyphenols in anticancer therapies?
several challenges in the clinical application of natural polyphenols, particularly their poor bioavailability due to rapid metabolism and low concentrations in the bloodstream. Artificial intelligence (AI) can help improve bioavailability in the following ways:
- Prodrug Development: AI can assist in designing prodrugs (compounds converted into an active drug form after administration) to enhance the absorption and stability of polyphenols. For example, derivatives of polyphenols like scutellarein have been shown to improve bioavailability. AI tools could help optimize the molecular design of such prodrugs by predicting the most effective structural modifications.
- Optimization of Delivery Systems: AI can model and optimize nanoparticle-based or other novel drug delivery systems that could protect polyphenols from rapid metabolism and allow them to reach their target sites more effectively. This could include AI-driven optimization of nanocarrier formulations that enhance stability, absorption, and target specificity.
- AI and Metabolism Predictions: AI can simulate and predict how polyphenols interact with intestinal enzymes and gut microbiota, which are key factors influencing bioavailability. By predicting these interactions, AI can guide the formulation of polyphenols to ensure more effective absorption in the gastrointestinal tract, overcoming challenges related to their complex metabolism.
Through these innovations, AI can help make polyphenol-based anticancer therapies more practical and effective by addressing one of their key limitations—bioavailability.
2. What are the challenges in translating preclinical findings of polyphenols to clinical success?
several key challenges in translating the promising anticancer effects of natural polyphenols from preclinical studies to clinical applications:
- Poor Bioavailability: As mentioned earlier, polyphenols generally have low bioavailability, meaning that they are rapidly metabolized and excreted, leading to insufficient concentrations in the bloodstream to exert meaningful therapeutic effects. Overcoming this hurdle requires significant optimization, often through formulation modifications or alternative delivery methods.
- Dosage and Toxicity: While polyphenols are abundant in the human diet and appear safe at low levels, high doses required for therapeutic effects can result in toxicity. Finding the right therapeutic dosage that balances efficacy without inducing harmful side effects remains a major obstacle. Preclinical models may not accurately predict human responses due to metabolic differences.
- Complex Metabolism and Interactions: The metabolism of polyphenols involves various enzymes and interactions with gut microbiota, which complicates their effectiveness and predictability in human systems. These interactions make it difficult to determine consistent dosing and therapeutic responses in clinical settings, as outcomes can vary widely between individuals.
- Insufficient Clinical Trials: While some polyphenols like resveratrol and curcumin have entered clinical trials, many studies are in early stages, and only a few have advanced to phases that assess their true therapeutic potential. Additionally, many trials combine polyphenols with conventional therapies, making it difficult to isolate their independent effects.
- Lack of Comprehensive Data: Translating preclinical findings to clinical success is often hindered by a lack of comprehensive clinical data on polyphenols, particularly in regard to long-term safety and efficacy. While AI tools can help bridge the gap by accelerating the research process, there is still a need for more large-scale clinical trials that focus on standalone polyphenol interventions.
These challenges underscore the need for more advanced research, better-designed clinical trials, and AI-driven innovations to optimize the application of polyphenols in cancer therapy.
3. How do specific polyphenols interact with the NF-κB signaling pathway to combat cancer?
natural polyphenols can inhibit the NF-κB signaling pathway, which is critical in regulating inflammation, cell proliferation, and cancer progression. Several polyphenols have been found to interfere with this pathway, leading to anticancer effects:
- Resveratrol: This well-known polyphenol inhibits the NF-κB pathway by suppressing its activation through multiple mechanisms. It blocks the phosphorylation and degradation of IκB proteins, which are crucial for preventing the nuclear translocation of NF-κB. This inhibition leads to the suppression of genes involved in tumor growth, metastasis, and inflammation, such as VEGF and ICAM.
- Curcumin: Curcumin blocks the activation of NF-κB by inhibiting IKK activity, which is essential for the phosphorylation of IκB proteins. By doing so, it prevents NF-κB from entering the nucleus and activating pro-tumor genes. Curcumin’s inhibition of NF-κB is linked to its ability to induce apoptosis and hinder cell proliferation in various cancers.
- Epigallocatechin-3-gallate (EGCG): Found in green tea, EGCG enhances the anticancer effect of other drugs, such as doxorubicin, by modulating the NF-κB/MDM2/p53 pathway. This results in reduced cancer cell survival and increased sensitivity to chemotherapy.
These polyphenols not only inhibit NF-κB but also modulate other signaling molecules downstream, amplifying their anticancer effects by blocking cell survival pathways, promoting apoptosis, and reducing metastasis and inflammation in cancer cells.
Conclusion
The integration of artificial intelligence in cancer research marks a new era in drug discovery. By enhancing the bioavailability, efficacy, and safety of natural polyphenols, AI is helping researchers overcome the challenges that have long hindered the use of these compounds in cancer therapy. As more breakthroughs emerge, it’s clear that AI will continue to play a vital role in developing polyphenol-based anticancer drugs that could revolutionize cancer treatment.
“AI is not just accelerating drug discovery, it’s reshaping the entire landscape of cancer therapy,” said Ying Zheng, lead author of the 2024 study.
Take control of your health and explore how natural polyphenols combined with cutting-edge AI technologies can offer new hope in the fight against cancer.
*Disclaimer: This summary/ long form blog was created with the assistance of AI language model ChatGPT by OpenAI.
WRITTEN BY
Social Chats
Social Chats is a multimedia and entertainment company. It’s a division of kNOw Aging, inc. a communications consultancy.









