
The Fight for Health Freedom in America
Health freedom in the USA has evolved dramatically over the past few decades, shaped by pivotal moments like the rise of AIDS buyers clubs in the 1980s and the enactment of the Dietary Supplement Health and Education Act (DSHEA) in 1994. These stories are captured in two key works: As We See It: When Vitamins Became Legal by Bill Faloon in the November 2024 issue of Life Extension Magazine, and “Managing Your Own Survival”: Buyers Clubs in the AIDS Epidemic by Grinnell College, published as part of Health & Medicine in American History. Both movements arose out of necessity, driven by the belief that the government’s response to pressing health needs was insufficient, leading individuals to take control of their health.
During the AIDS epidemic, buyers clubs emerged as a response to the lack of effective, accessible treatments. These grassroots organizations provided a lifeline, delivering non-FDA-approved drugs to thousands of members who felt abandoned by the slow response of government agencies like the FDA and CDC. The story of these buyers clubs shares common threads with the later push for supplement freedom, which led to the passage of DSHEA. This legislation revolutionized access to vitamins and supplements in the United States. Together, these events highlight the importance of self-determination and consumer choice in health care.
Mind Map of Evolution of Health Freedom:
Summary:
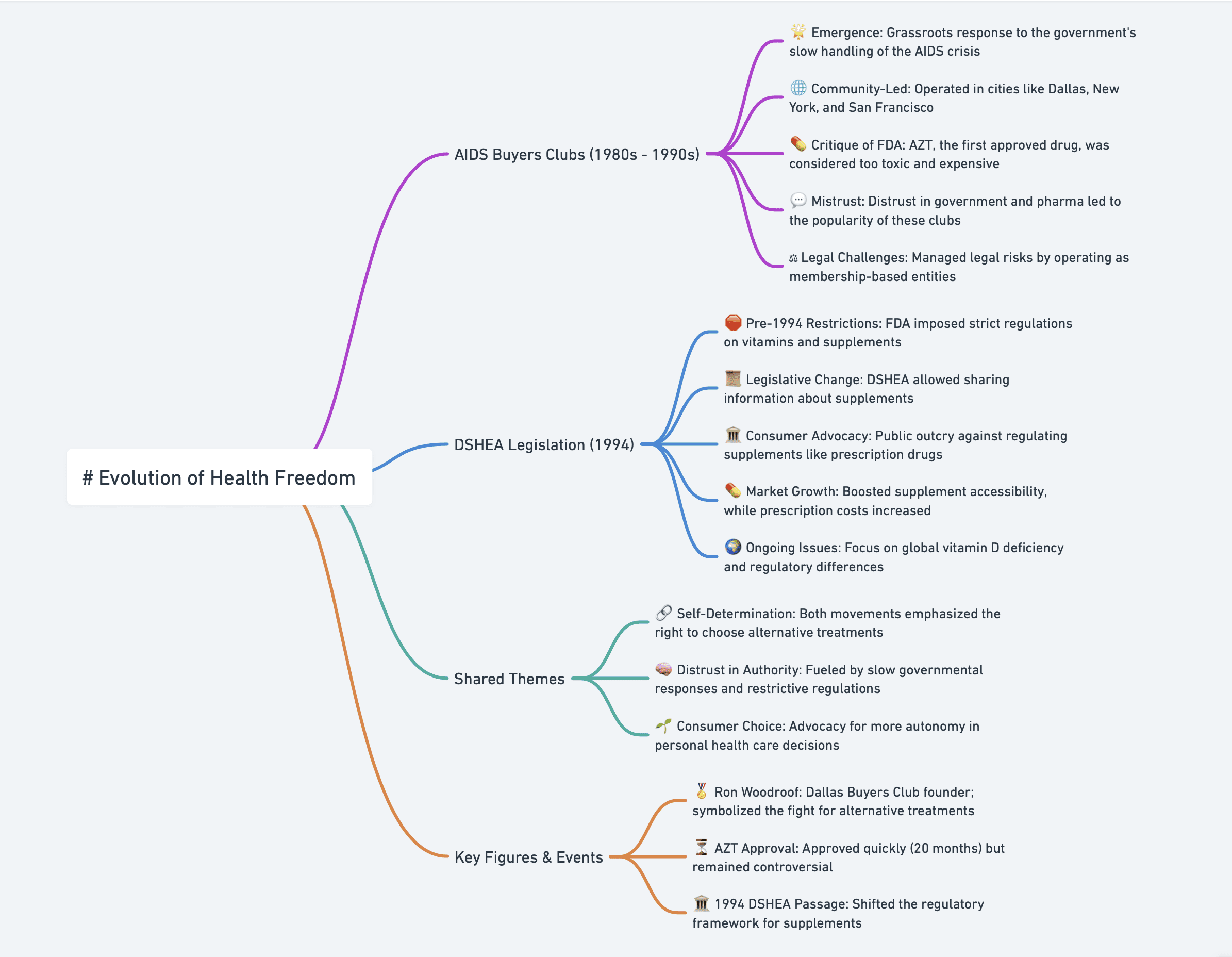
Managing Your Own Survival: Buyers Clubs in the AIDS Epidemic
Time Interval: 1980s – 1990s
- 🚨 Emergence of Buyers Clubs: In the 1980s and 1990s, during the AIDS epidemic, buyers clubs emerged as a grassroots response to the government’s slow action in addressing the crisis.
- 🌐 Community-Led Efforts: Buyers clubs operated in major U.S. cities like Dallas, New York, and San Francisco. They functioned as independent entities, sharing resources and information.
- 💊 Critique of FDA and AZT: The first FDA-approved drug, AZT, was introduced in 1987, but many found it too toxic and costly, leading patients to seek alternatives.
- 💬 Mistrust in Authorities: Distrust in the government’s slow response and the pharmaceutical industry fueled the popularity of these clubs. Many saw these organizations as a means to regain control of their treatment.
- ⚖️ Legal and Ethical Controversies: Despite criticisms from some in the medical community, buyers clubs became a symbol of self-determination, offering access to potentially life-saving drugs.
As We See It: When Vitamins Became Legal
Time Interval: 1994 – 2024
- 🛑 Pre-1994 Restrictions: Prior to October 1994, the FDA imposed strict regulations on vitamins, treating them like unapproved drugs.
- 📜 DSHEA Enactment: The Dietary Supplement Health and Education Act (DSHEA) of 1994 transformed the regulatory landscape, allowing the dissemination of truthful, non-misleading information about supplements.
- 🏛️ Public Outcry & Legislative Change: Consumer backlash against potential reclassification of supplements as prescription drugs led to legislative action.
- 💊 Market Impact: After DSHEA, the supplement industry grew, making supplements more affordable while prescription drug prices increased.
- 🌍 Global Vitamin D Concerns: The document highlights ongoing global issues with vitamin D deficiency, emphasizing the inadequacy of some national regulations.
The Birth of AIDS Buyers Clubs: Self-Determination in Action
The AIDS epidemic of the 1980s and 1990s devastated communities across the United States, with government agencies struggling to provide timely solutions. As detailed in “Managing Your Own Survival” by Grinnell College, the slow governmental response led to the rise of buyers clubs—community-driven initiatives that smuggled non-FDA-approved drugs from abroad. This allowed those with HIV/AIDS to access treatments not available through traditional channels, often at a lower cost than FDA-approved options like AZT.
In 1992, Bill Minutaglio, a reporter for the Dallas Morning News, delved into the story of these buyers clubs that were providing a lifeline for AIDS patients across the country. He tracked down Ron Woodroof, founder of the Dallas Buyers Club, for a feature. According to Minutaglio’s original article, many clubs—including the one in Dallas—took measures to ensure safety by sending smuggled drugs to local labs for purity testing, often after importing them from Mexico. Despite the Dallas Buyers Club’s reputation for distributing highly experimental treatments, Woodroof’s determination was clear. As he told Minutaglio, “Dammit … I don’t see how anything can be more toxic than HIV itself. I have taken chances that have almost killed me and I will keep on taking them. I have nothing to lose.” Woodroof had founded the club in 1987, and at its peak, it provided a lifeline to as many as 4,000 regular members.
DSHEA and the Fight for Supplement Access
The same themes of self-determination and frustration with regulatory barriers appeared again in the early 1990s, as consumers fought for access to dietary supplements, a story chronicled in As We See It: When Vitamins Became Legal. Before DSHEA, the FDA’s heavy-handed approach led to company raids and restricted access to many supplements. The passage of DSHEA was driven by consumer activism, which demanded the freedom to choose and manage personal health care, much like the AIDS buyers clubs before it.
DSHEA fundamentally changed the regulatory landscape for vitamins and supplements in the USA. It allowed consumers to access a wide range of supplements without needing prescriptions and ensured that truthful, non-misleading information could be shared about these products. This legislative change was driven by a grassroots movement that demanded the freedom to choose and manage their own health care—echoing the spirit of the AIDS buyers clubs.
Shared Lessons: Trust, Choice, and Health Care
Both the buyers clubs and DSHEA advocates shared a belief in the right to access alternative treatments. Whether it meant distributing non-FDA-approved drugs for AIDS patients or advocating for unrestricted access to supplements, the two movements revealed a shift in consumer attitudes towards health care. The result was a new era of health freedom that emphasized individual choice and access to information.
Diagram: Health Freedom Evolution
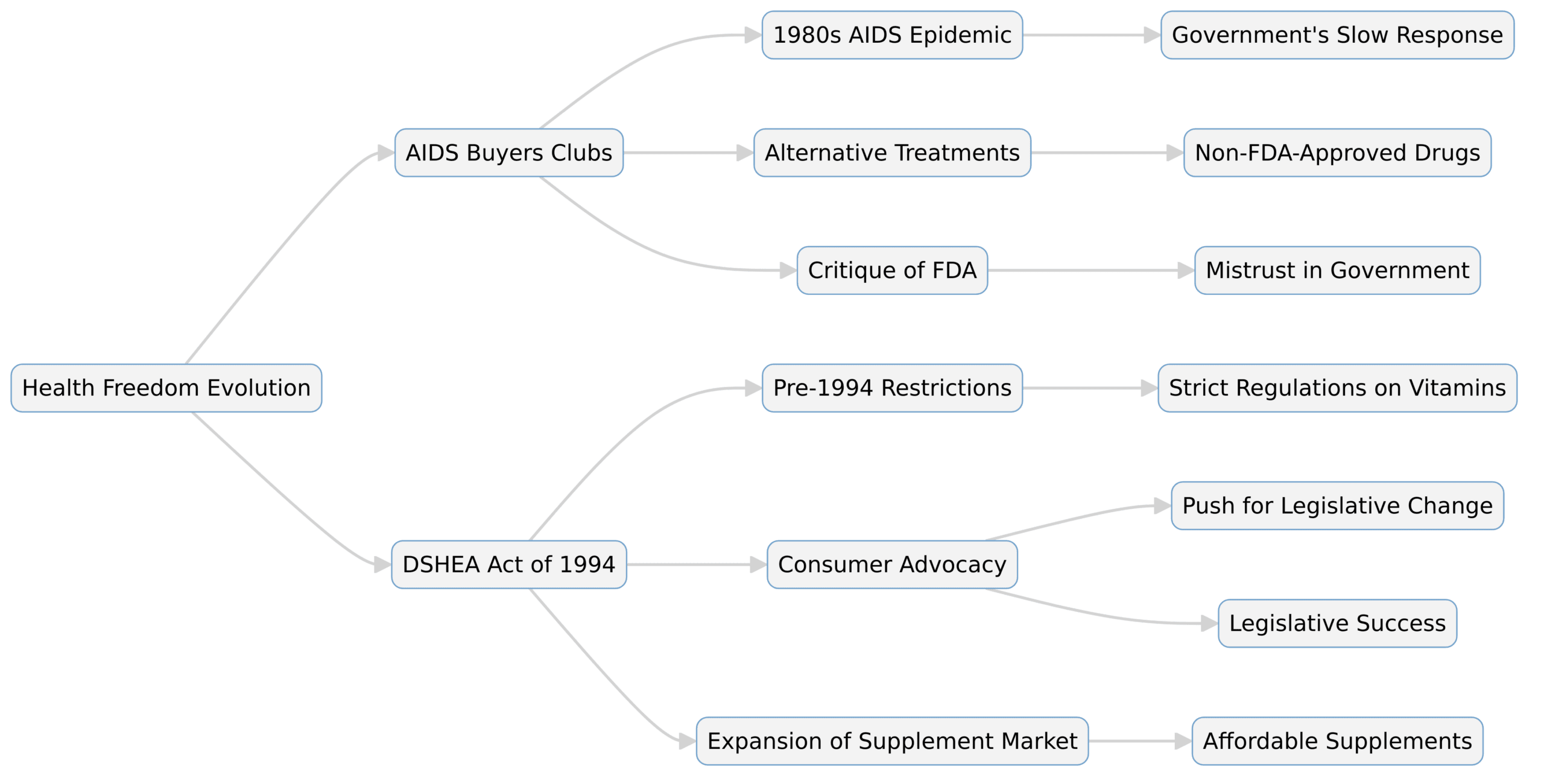
This diagram outlines the key phases: the rise of AIDS buyers clubs, the impact of DSHEA, and the shared lessons from these movements, highlighting their collective influence on health freedom and regulatory changes.
Insights Based on Numbers
- 📅 Years of Inaction: It took the CDC several years to identify the transmission methods and cause of AIDS, with official recognition of HIV only in the mid-1980s.
- 💊 Rapid Approval vs. Usual Process: AZT was approved in just 20 months—much faster than the typical 10-year approval process—indicating the pressure to find a treatment, yet it was insufficient for many.
- 💵 30 Years of DSHEA: DSHEA has allowed for consumer access to a wide range of supplements without prescriptions, contributing to a more empowered market. This contrasts with ongoing restrictions in other countries, especially concerning vitamin D dosage regulations.
Explanatory Q&A
1. What role did consumer activism play in the passing of the DSHEA in 1994?
Consumer activism played a crucial role in the passing of the Dietary Supplement Health and Education Act (DSHEA) in 1994. Before its enactment, the FDA had the authority to restrict and even seize supplements, leading to situations where companies faced raids and owners risked arrest. This harsh regulatory approach prompted significant backlash from the public.
As the FDA moved to reclassify many dietary supplements as prescription drugs, consumers became increasingly concerned about losing access to these products. This led to a flood of letters and appeals to Congress, urging them to protect the public’s right to purchase and access supplements freely. The widespread consumer mobilization effectively pushed legislators to curb the FDA’s powers, culminating in the passage of DSHEA. This law ensured that consumers could access a wide range of supplements while preventing the FDA from regulating truthful, non-misleading information about these products.
2. How has the perception of dietary supplements evolved since the enactment of DSHEA?
Since the enactment of the Dietary Supplement Health and Education Act (DSHEA) in 1994, the perception of dietary supplements has shifted significantly, both among consumers and within the regulatory framework. Initially, supplements were viewed with skepticism and subjected to strict control, with the FDA considering many supplements as unapproved drugs. This created an environment of fear among both companies and consumers, with concerns about sudden removal of products from the market.
However, DSHEA’s passage marked a turning point by redefining dietary supplements as a separate category from pharmaceuticals, allowing for more freedom in their production, sale, and the sharing of information about their benefits. This shift enabled the supplement industry to expand rapidly, leading to increased public awareness and acceptance of the role of supplements in maintaining health and wellness.
Over the past three decades, supplements have become mainstream, with a large portion of the American population using them regularly. The narrative has evolved from skepticism to widespread acceptance, driven by increased scientific research and consumer interest in natural health solutions. Supplements like vitamin D, omega-3s, and coenzyme Q10 are now commonly recognized for their benefits, and access to reliable information about their use has become a cornerstone of this transformation. Moreover, while concerns about safety initially dominated the discourse, the lack of widespread adverse health effects has further solidified the positive perception of supplements as an accessible means of supporting health.
3. What are the differences in vitamin D regulations between the U.S. and other countries?
The regulations surrounding vitamin D supplements differ significantly between the United States and many other countries, primarily in terms of dosage limits and classification.
- United States: After the passage of the DSHEA in 1994, dietary supplements, including vitamin D, have been more freely available to consumers without needing a prescription. The law allows the dissemination of information about these supplements, provided it is truthful and not misleading. As a result, high-potency vitamin D supplements, such as those containing 2000 IU or more, are readily available in the U.S. Many consumers take advantage of these options to maintain optimal vitamin D levels, particularly since research has highlighted the benefits of higher doses for health.
- Other Countries: In contrast, many countries outside the U.S. have stricter regulations regarding vitamin D supplements. These regulations often classify supplements as drugs if they exceed certain dosage thresholds, typically between 400 and 800 IU per day. For instance, in parts of Europe, higher doses of vitamin D may require a prescription, making it more challenging for consumers to access the amounts needed for optimal blood levels. These stricter regulations are often based on a more conservative interpretation of safety and efficacy data.
- Health Implications: The document points out that these limitations can contribute to widespread vitamin D deficiencies in regions where access to higher-dose supplements is restricted. Research cited in the document suggests that many people worldwide have 25-hydroxyvitamin D levels below the minimum recommended threshold, with some populations having dangerously low levels. The 2024 review mentioned advocates for a universal daily dose of 2000 IU as a more effective approach to addressing these global deficiencies, contrasting with the lower limits imposed in many countries.
The disparity between the U.S. and other nations reflects different regulatory philosophies—one that prioritizes consumer freedom and access, and another that emphasizes a more cautious, prescription-based approach.
4. How did buyers clubs navigate legal challenges while providing unapproved drugs to members?
Buyers clubs faced significant legal challenges as they operated outside of the FDA’s regulatory framework, providing unapproved drugs to people with HIV/AIDS. Their activities often involved smuggling medications from other countries where these treatments were available but not yet approved in the United States. This put them at odds with U.S. law, as the FDA was responsible for ensuring that all drugs distributed in the country met safety and efficacy standards.
Despite the risks, buyers clubs found ways to navigate these challenges. They structured themselves as membership organizations, where members paid fees to access the treatments. By presenting themselves as providing information and products for personal use rather than commercial sales, they aimed to skirt around stricter regulations meant for for-profit pharmaceutical distribution.
In some instances, medical professionals indirectly supported these clubs. For example, some doctors would discreetly provide information about the existence of these clubs to patients, hinting at alternative options for obtaining experimental treatments that were not legally accessible through traditional channels.
The buyers clubs also established their own testing protocols for the drugs they distributed. Although not regulated by the FDA, these clubs often sent imported drugs to labs for purity testing, aiming to assure their members of the safety of the treatments provided. This self-regulation was a key defense against criticisms that they were distributing unsafe or impure substances.
However, their operations were controversial. Some within the medical community, like Dr. Chalmers, strongly opposed buyers clubs, arguing that unregulated access to these drugs could lead to harm through improper use or unknown side effects. Nevertheless, the urgency of the AIDS crisis and the lack of timely official treatments created a space where many people with HIV/AIDS felt compelled to take these risks, seeing the buyers clubs as their best chance at survival when facing a disease with few alternatives.
5. What role did the distrust of pharmaceutical companies play in the popularity of buyers clubs?
The distrust of pharmaceutical companies and the government’s handling of the AIDS crisis played a significant role in the rise and popularity of buyers clubs. Many people with HIV/AIDS felt that the official response to the epidemic was slow and inadequate, leading to a deep mistrust of the institutions responsible for their care.
Key factors contributing to this distrust included:
- Delayed Recognition and Response: The government, particularly the CDC and FDA, took years to acknowledge the full scale of the AIDS epidemic and to establish guidelines around its transmission and treatment. This delay, alongside the slow progress in approving effective treatments, fueled feelings of abandonment among those affected by HIV/AIDS.
- AZT’s Limitations: When AZT became the first FDA-approved drug for AIDS in 1987, it was seen as a limited solution. While it offered some hope, AZT was highly toxic, had significant side effects, and came at an extremely high cost, making it inaccessible or unsuitable for many patients. This situation reinforced perceptions that pharmaceutical companies were profiting from a life-threatening crisis rather than prioritizing patient needs.
- Perceived Profit Motives: There was a widespread belief among people with AIDS that the government and pharmaceutical companies were restricting access to potentially beneficial drugs to maintain market control and maximize profits. This led to accusations that these entities were “playing God” with the lives of those affected by the epidemic, manipulating the market to benefit from the scarcity of available treatments.
- Self-Determination in Health Decisions: This era was marked by a broader cultural shift towards skepticism of authority and a desire for greater self-determination, particularly in healthcare. People with AIDS saw buyers clubs as a means to regain control over their treatment, bypassing the perceived bureaucracy and profit motives of pharmaceutical companies. These clubs represented an alternative path to accessing drugs that might offer hope, even if they were not officially sanctioned.
The popularity of buyers clubs can thus be understood as a response to the perceived failures and profit-driven motives of both the pharmaceutical industry and the federal government. For many, joining these clubs was not just a practical decision for accessing treatments but also a symbolic act of defiance against a system they believed had failed them.
6. How did the buyers clubs’ efforts compare with government initiatives to address the AIDS epidemic?
The efforts of buyers clubs and government initiatives to address the AIDS epidemic diverged significantly, reflecting contrasting approaches to providing care for people with HIV/AIDS during the crisis.
- Government Initiatives:
- Slow Response and Bureaucratic Processes: The U.S. government’s response to the AIDS crisis in the 1980s and early 1990s was widely criticized for being slow and inadequate. It took years for the CDC to identify AIDS as a distinct condition, understand its transmission methods, and recognize HIV as its cause. This slow pace of action delayed public health campaigns and the development of effective treatments.
- FDA’s Drug Approval Process: The Food and Drug Administration (FDA) typically follows a lengthy process for drug approval, which can take up to 10 years. Under pressure during the AIDS epidemic, the FDA expedited the approval of AZT in 1987, a move that was faster than usual but still insufficient for the urgent needs of many patients. Despite this, AZT had severe side effects and was prohibitively expensive for many, limiting its accessibility.
- Limited Information and Support: The government’s public health efforts initially provided little in the way of clear guidance or support for people with HIV/AIDS. Information about how to manage and treat the condition was scarce, contributing to a sense of abandonment and helplessness among those affected.
- Buyers Clubs’ Approach:
- Grassroots Organization and Direct Action: Buyers clubs arose as community-led initiatives, driven by people with HIV/AIDS and their advocates. These clubs bypassed the lengthy FDA approval process by sourcing drugs from international markets that had not been approved for use in the U.S. This allowed members to access experimental or alternative treatments much more quickly.
- Focus on Accessibility and Affordability: Unlike government programs and pharmaceutical companies, many buyers clubs prioritized making treatments affordable. They often operated under ethical guidelines, such as the Ft. Lauderdale Principles, which emphasized providing drugs at the “lowest possible cost” to those in need.
- Self-Regulation and Community Trust: While unregulated by the FDA, buyers clubs took steps to test the purity of the drugs they distributed, building a sense of trust within their communities. They provided not only physical treatments but also a sense of agency and hope, offering members a way to actively manage their own survival in the face of a life-threatening illness.
- Impact on the AIDS Epidemic:
- The two approaches reflected differing priorities and values. Government initiatives focused on maintaining regulatory standards and protecting public health through established procedures, even if these were slow. In contrast, buyers clubs prioritized speed and access, often at the expense of formal regulation, in response to the urgent needs of people with HIV/AIDS.
- Buyers clubs became a lifeline for many who felt that government measures were insufficient, offering a form of resistance against the perceived neglect and indifference of public institutions. They filled a critical gap by providing access to treatments when official channels failed to meet the needs of those living with HIV/AIDS.
Overall, buyers clubs embodied a more immediate, patient-centered approach, while the government response remained constrained by formal protocols. This divergence highlighted the tension between patient autonomy and regulatory oversight during a public health crisis, illustrating the ways in which communities can mobilize to address urgent health needs when institutional support falls short.
Conclusion
The intertwined history of AIDS buyers clubs and the passage of DSHEA underscores a fundamental truth: when faced with life-threatening challenges, people will find ways to take control of their own health. Whether through underground networks distributing experimental treatments or through legislative advocacy for supplement access, these movements have reshaped the landscape of health freedom in the USA.
As Ron Woodroof, a founder of one of the most famous buyers clubs, once said, “Dammit … I don’t see how anything can be more toxic than HIV itself. I have taken chances that have almost killed me and I will keep on taking them. I have nothing to lose.” This sentiment captures the essence of why people fought so fiercely for health freedom—when the stakes are high, the power to choose becomes a matter of survival.
Similarly, the passage of DSHEA in 1994, as detailed in November 2024 edition of Life Extension Magazine, represented a triumph for those who believed in the right to access and share information about supplements. It allowed Americans to maintain control over their own health, ensuring that the lessons learned from the AIDS crisis would continue to benefit those seeking alternative paths to well-being.
Call to Action:
💡 Take control of your health and stay informed! Subscribe to Life Extension Magazine to learn more about the history of health freedom and how you can advocate for your right to access the supplements and treatments that matter most. Use Code: TSZ23 to claim your FREE 12 month subscription.
Interactive Quiz: Assess Your Knowledge
*Disclaimer: This summary/ long form blog was created with the assistance of AI language model ChatGPT by OpenAI.
WRITTEN BY
Social Chats
Social Chats is a multimedia and entertainment company. It’s a division of kNOw Aging, inc. a communications consultancy.









